Electrochemical recycling is an efficient process that uses electricity to recover metals from electronic waste (e-waste). With e-waste growing by 3–5% annually and containing valuable metals like gold, copper, and rare earth elements, traditional methods such as smelting and chemical leaching are falling short due to high emissions and waste. Electrochemical methods offer a cleaner, lower-energy alternative, achieving high recovery rates while reducing toxic byproducts.
Key points about electrochemical recycling:
- What It Does: Recovers metals like copper, gold, and palladium from e-waste using electric currents in electrolytic cells.
- How It Works: Metals are dissolved through chemical leaching, oxidants are regenerated, and pure metals are deposited on electrodes.
- Efficiency: Recovers over 90% of base metals and reduces energy use by up to 50% compared to traditional methods.
- Environmental Impact: Cuts chemical waste, eliminates combustion emissions, and reduces reliance on mining.
This process aligns with the growing need to recycle high-value materials from e-waste, providing both economic and environmental benefits while supporting a circular economy.
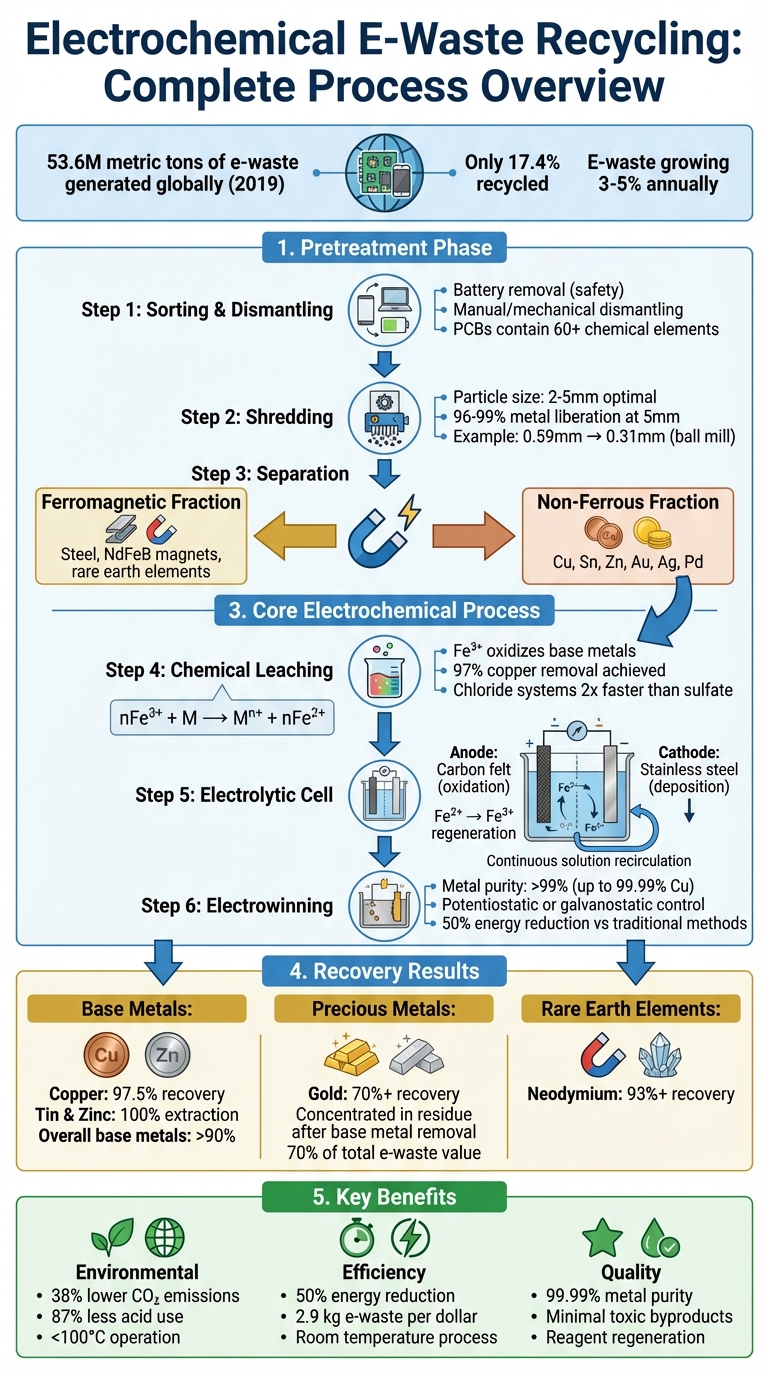
Electrochemical E-Waste Recycling Process: From Sorting to Metal Recovery
E-RECOV, Electrochemical Recycling Electronic Constituents of Value
E-Waste Pretreatment Methods
Before diving into electrochemical recycling, e-waste must first go through a pretreatment process. This step separates metals from plastics, ceramics, and resins, making the recycling process more efficient, cost-effective, and environmentally friendly. Without this crucial step, the complex mix of materials in e-waste would make it nearly impossible to extract metals selectively.
"Effective sorting and pre-treatment of e-waste are very critical to overcome downstream processing challenges and costs." – Varun Rai, Researcher, Nanyang Technological University [8]
The pretreatment process results in two main material streams: a ferromagnetic fraction, which includes steel and rare earth magnets, and a non-ferrous fraction, rich in valuable metals like copper, gold, and silver.
Sorting and Dismantling
The first step in pretreatment involves sorting e-waste based on its metal content and physical characteristics. This helps determine the best treatment methods [4]. Batteries are removed early in the process to prevent safety risks and contamination during mechanical processing.
After battery removal, devices are dismantled – either manually or with machinery – to separate key components like casings, circuit boards, and large metal parts. Special attention is given to printed circuit boards (PCBs), which contain over 60 different chemical elements and are a primary source of recoverable metals [5].
For example, researchers at the Idaho National Laboratory developed a detailed pretreatment process for mobile electronics. Their method started with battery removal and sorting, followed by shredding phones to a particle size of 0.59 mm. Magnetic separation was then used to extract ferrous materials, and the remaining non-ferrous materials were further reduced to 0.31 mm using a ball mill. This approach achieved an impressive 97.5% copper recovery efficiency [2].
Shredding and Size Reduction
Once sorted and dismantled, e-waste is mechanically shredded into smaller pieces. This size reduction is critical because it liberates metals from non-metal substrates, making them easier to recover. Research shows that most metals are freed when particles are reduced to sizes between 2 mm and 5 mm. For PCBs, 96–99% of metals are released at around 5 mm [7].
Shredding also increases the surface area available for chemical reactions during electrochemical recovery. This enhanced surface area improves the speed and efficiency of extraction processes, allowing for higher solid-to-liquid ratios and reducing the amount of chemicals needed. Facilities use different shredding techniques depending on their specific processes. For instance, one site in Spain employs a "super pre-chopper" followed by a ring shredder, while a New Zealand facility mills e-waste to a "sand-like consistency" before mixing it with a proprietary leaching solution [7]. These methods ensure optimal preparation for the next stages of recycling.
"The use of a packed bed column improved the contact between the leaching solution and the e-waste, allowing the potential to completely deplete the oxidant through a single pass, increasing the S/L ratio." – Luis A. Diaz et al., Idaho National Laboratory [2]
Magnetic and Electrostatic Separation
After shredding, magnetic separation is used to extract the ferrous fraction, which includes steel and NdFeB magnets, from the non-ferrous materials [2]. This step creates two distinct processing paths: the ferromagnetic fraction, which is processed for rare earth elements like neodymium (commonly found in electronic vibrators and speakers), and the non-ferrous fraction, which contains base and precious metals such as copper, gold, and palladium.
Magnetic separation plays a key role in recovering critical materials that contribute to a sustainable recycling system. By isolating these components early, specialized techniques can be applied to recover rare earth elements more efficiently. Electrostatic separation, which takes advantage of differences in electrical conductivity between metals and non-metallic materials like plastics and ceramics [6], further purifies the non-ferrous stream. Together, these methods ensure that only metal-rich materials proceed to the electrochemical recovery stage, reducing chemical waste and improving overall efficiency.
| Separation Fraction | Target Materials | Typical Recovery Method |
|---|---|---|
| Ferromagnetic Fraction | Steel, NdFeB Magnets, Rare Earth Elements | Anaerobic acid extraction, precipitation |
| Non-Ferrous Fraction | Copper, Tin, Zinc, Gold, Silver, Palladium | Electrochemical recovery, electrowinning, leaching |
This content is for informational purposes only. Consult official regulations and qualified professionals before making sourcing or formulation decisions.
Core Electrochemical Process Steps
Following sorting and shredding, the electrochemical process extracts and purifies metals from e-waste under room temperature conditions using reusable reagents. This process is divided into three key steps: chemical leaching, which dissolves metals into a solution; electrolytic regeneration, which restores the leaching agent while recovering metals; and controlled deposition, where high-purity metals are plated onto the cathode. These steps work in harmony to ensure efficient metal recovery.
Chemical Leaching and Oxidation
To recover metals electrochemically, they first need to be dissolved from solid e-waste into a liquid electrolyte. This is achieved through oxidative leaching, where a weak oxidizer – commonly ferric ions (Fe³⁺) – reacts with base metals, converting them into a soluble ionic form. The reaction can be summarized as:
nFe³⁺ + M → Mⁿ⁺ + nFe²⁺,
where M represents metals like copper, tin, zinc, nickel, or lead.
Ferric ions are selective, targeting base metals while leaving precious metals such as gold and palladium intact in the solid residue. Two common leaching systems are used: a chloride matrix (hydrochloric acid with ferric chloride) and a sulfate matrix (sulfuric acid with ferric sulfate). Research highlights that chloride-based solutions dissolve metals nearly twice as quickly as sulfate-based ones [2].
For instance, experiments at Idaho National Laboratory using FeCl₃ in an HCl matrix achieved 97% copper removal and complete extraction of tin and zinc from shredded cell phones. This process also enriched the remaining residue with precious metals like gold and palladium [2].
"The main advantage of this process is that it increases the concentration of gold and palladium in the waste material by the selective and complete removal of the base metals with minimal chemical input." – Luis A. Diaz et al., Idaho National Laboratory [2]
Once leaching is complete, the solution is transferred to an electrolytic cell for further processing, where regeneration and metal deposition occur.
Electrolytic Cell Setup
An electrolytic cell is designed with three main components: an anode for oxidation, a cathode for metal deposition, and an electrolyte to transport ions. This setup allows for continuous regeneration of reagents and extraction of metals [8].
In a flow-cell configuration, the electrolyte circulates between a packed bed of shredded e-waste and the electrolytic cell. The solution dissolves metals as it passes through the e-waste, then moves to the cell where Fe²⁺ is oxidized back to Fe³⁺ at the anode, while metal ions are deposited onto the cathode. The regenerated solution is then recirculated to repeat the process.
For example, Idaho National Laboratory employed a trough flow-cell with a 304 stainless steel mesh cathode and a carbon felt anode. Using this setup, they achieved over 90% copper extraction in both sulfuric and hydrochloric acid systems [2].
Electrode materials play a critical role in the process. Cathodes are typically made from stainless steel mesh or titanium, offering excellent electrical conductivity and a suitable surface for metal deposition. Anodes, often made from graphite or carbon felt, resist corrosion during oxidation. The choice of electrolyte – aqueous, organic, or ionic liquid – depends on the metals being recovered. Aqueous electrolytes are favored for base metals like copper and zinc due to their low cost and high ionic mobility. However, sulfate-based systems can face challenges like anode fouling, where tin dioxide (SnO₂) forms on the anode, increasing voltage and hindering Fe³⁺ regeneration. This issue is less common in chloride-based systems [2].
With the cell optimized for efficient ion flow, the process moves to electrowinning for metal recovery.
Electrochemical Reactions and Metal Deposition
Electrowinning is the final step, where high-purity metals are deposited onto the cathode. When an electrical potential is applied, positively charged metal ions migrate to the cathode, gain electrons, and form solid metal deposits. This process ensures the recovery of metals in their purest form [8].
Two common control methods are used: potentiostatic control (constant voltage), which enables selective recovery of specific metals based on their reduction potentials, and galvanostatic control (constant current), preferred for industrial applications due to its consistent energy usage [8].
"Electrochemical approaches in metal recovery have several advantages such as uniformity in metal deposition, high purity, automation, easy control, cost effectiveness, and relatively fast processing time." – Varun Rai et al., Nanyang Technological University [8]
Electrowinning can produce copper with a purity of up to 99.99%, far exceeding the quality achieved through chemical precipitation or smelting. By using the Fe²⁺/Fe³⁺ oxidation reaction at the anode instead of the oxygen evolution reaction, energy consumption can be reduced by as much as 50% compared to traditional methods [2]. These benefits make electrochemical recovery a practical choice for small to medium-scale operations.
This content is for informational purposes only. Consult official regulations and qualified professionals before making sourcing or formulation decisions.
Key Technologies and Process Improvements
Advancements in technology are transforming metal recovery processes, focusing on two primary objectives: selective metal extraction and reducing operating costs. Some of these methods operate at room temperature, while others rely on rapid heating or specialized membranes to achieve their goals. Below, we explore electrodeposition techniques and energy-efficient improvements that enhance these processes.
Electrodeposition and Electrodialysis
Electrodeposition involves controlling electrical potential to selectively recover metals. Copper is often the first target due to its reduction potential (+0.34 V vs. SHE), making it easier to deposit compared to base metals like zinc or nickel. By fine-tuning the voltage, copper can be plated onto the cathode, leaving other metals in solution for later recovery [11].
Electrodialysis (EED) takes this process further by incorporating ion-exchange membranes into the system. These membranes allow metal ions to pass through while blocking other compounds, enabling the recovery of metals and the recycling of reagents like ammonia. A pilot study conducted in September 2021 by researchers Javier Llanos, Yelitza Delgado, and Francisco J. Fernández-Morales at the University of Castilla-La Mancha demonstrated promising results. Using stainless steel and brass cathodes, they achieved 100% copper removal in the lab and 40% at the pilot scale, with energy consumption at 2 kWh per kilogram of copper [11]. EED systems have also shown nearly 80% current efficiency in recovering copper from ammonia solutions [11].
Innovative reactor designs, such as jet electrodeposition and cyclone cells, further enhance recovery rates by increasing electrolyte flow. These setups enable higher current densities (up to 12 A/dm²) with an efficiency of 76.3% [11]. By preventing side reactions, they can produce metal purities exceeding 99.9%, which is essential for applications like electronics manufacturing [11]. These advanced techniques are integral to improving both the precision and energy efficiency of electrochemical recycling.
Energy and Process Efficiency
Energy consumption remains a major factor in the economic feasibility of electrochemical recycling. Traditional methods often require high temperatures and extended processing times, but newer technologies are cutting both dramatically. Flash Joule Heating (FJH), for example, completes metal recovery in just 300 milliseconds. By applying 150 V at 3.73 kW/g, FJH achieved recovery rates of 94.2% for cobalt and 96.3% for lithium from LiCoO₂, using only 0.31 kWh per kilogram – significantly less energy than conventional methods [10].
Countercurrent leaching is another energy-efficient technique that concentrates target metals, reducing the volume of spent solutions. This approach lowers waste generation and cuts operating costs [12]. When combined with mediated electrochemical oxidation (MEO), which regenerates Fe³⁺, the process selectively recovers base metals while preserving precious metals for later extraction [9]. This step-by-step method minimizes the use of reagents and energy, making it a practical option for small to medium-scale operations. Together, these innovations enhance both the economic and environmental viability of metal recycling.
This content is for informational purposes only. Always consult official regulations and qualified professionals for sourcing and formulation decisions.
sbb-itb-aa4586a
Advantages, Limitations, and Comparisons
Examining the details of recycling methods reveals where electrochemical recycling shines and the hurdles it must overcome.
Comparison of Recycling Methods
Electrochemical recycling stands out when stacked against traditional methods like pyrometallurgical and hydrometallurgical approaches. The table below highlights key differences, focusing on operating conditions, energy use, environmental impact, and costs.
| Feature | Pyrometallurgical | Hydrometallurgical | Electrochemical |
|---|---|---|---|
| Operating Temperature | Very High (over 1,000°C / over 1,832°F) [13] | Moderate (<100°C / <212°F) [13] | Low (<100°C / <212°F) [14] |
| Energy Consumption | 420–1,120 KJ/kg [13] | Moderate [13] | 26% lower than pyrometallurgy [14] |
| CO₂ Emissions | High (1.3–3.8 kg/kg) [13] | Moderate (1.9–3.6 kg/kg) [13] | 38% lower than pyrometallurgy [14] |
| Chemical Use | Low (primarily heat) | High (acids/reagents) [14] | Very Low (87% less acid) [14] |
| Metal Purity | Moderate (requires refining) | High | Very High (>99%) [14] |
| Capital Cost | Very High (~$15M for 30 kt/yr) [5] | Moderate | Moderate (~$6.8M for 20 kt/yr) [15] |
| Operating Cost | High (energy intensive) [15] | $39–$64 per kg [13] | Low (lower energy/reagent use) [15] |
This comparison highlights why electrochemical recycling is gaining attention, despite the challenges it faces.
Advantages of Electrochemical Recycling
Electrochemical recycling offers a combination of high efficiency and reduced environmental impact. It achieves exceptional metal purity levels – over 99% for copper and lithium carbonate, and around 95% for cobalt and nickel [14][4][5].
The environmental benefits are equally noteworthy. For example, electrochemical processes cut hydrochloric acid use by 87% and reduce water consumption by 26% compared to hydrometallurgical methods [14]. They also lower greenhouse gas emissions by 38% relative to pyrometallurgical techniques, while operating at temperatures below 100°C (212°F). This eliminates the need for energy-intensive furnaces, which are a major source of toxic air pollutants [6][15].
"Fully electrochemical recycling methods are emerging as solutions to combat both cost and environmental challenges in battery recycling innovation." – Green Li-ion [14]
These benefits make electrochemical recycling an attractive option for industries seeking greener and more efficient recycling solutions.
Limitations and Challenges
Despite its advantages, electrochemical recycling is not without its obstacles. The upfront cost of approximately $6.8 million for a 20 kt/year facility can be prohibitive for smaller operations [15]. Additionally, the complex composition of e-waste makes selective metal recovery difficult. Similar reduction potentials among different metals can result in co-deposition, reducing product purity [15].
Operational challenges also arise from the use of highly acidic or alkaline solutions, which can corrode equipment and foul membranes, leading to higher maintenance costs and downtime [15][6]. Energy efficiency suffers from side reactions, such as hydrogen evolution, which divert current away from metal recovery. Still, advancements have improved energy consumption to 1.94 kWh/kg while achieving a 93% metal recovery rate [16].
These challenges highlight the need for ongoing improvements to enhance the reliability and efficiency of electrochemical recycling systems.
This content is for informational purposes only. Always consult official regulations and qualified professionals for sourcing and formulation decisions.
Metal Recovery Applications
Electrochemical recycling breathes new life into e-waste, turning it into valuable materials that feed diverse manufacturing industries. By recovering both common base metals and rare, high-value precious metals, this process helps bridge the gap between waste and resource efficiency.
Recovery of Base and Precious Metals
Base metals like copper, tin, nickel, and zinc are among the most commonly recovered materials from e-waste, especially from circuit boards and wiring. Copper, in particular, stands out due to its abundance, making it a key material reintroduced into production supply chains. For instance, printed circuit boards, which are composed of roughly 30% metal by weight, demonstrate impressive copper recovery rates of up to 97.5% [5\].
On the other hand, precious metals such as gold, silver, and palladium – though present in smaller amounts – account for over 70% of the total value of e-waste like cell phones, calculators, and circuit board scraps [1]. These metals are highly sought after in electronics manufacturing because of their excellent conductivity and resistance to corrosion.
"The highest recovery value comes from metals with the lowest concentration. A small percentage of gold is found in mobile electronics, and yet it contributes to the highest portion of the total recoverable value of e-waste."
– Luis A. Diaz et al., Idaho National Laboratory [2]
In addition to these, rare earth elements like neodymium, often found in magnets, play a critical role in industries such as energy infrastructure. Electrolysis methods can recover these materials at rates exceeding 93% [4]. Similarly, platinum group metals are reclaimed for use in high-conductivity components like switching contacts, relays, and sensors, vital for telecommunications and industrial electronics [3\].
While the types of metals recovered are essential, the efficiency of the recycling process is equally impressive.
Recycling Rates and Efficiency
Modern electrochemical recycling systems deliver exceptional recovery rates. For example, a 2016 study at Idaho National Laboratory revealed a process for mobile electronics that achieved 97.5% copper recovery while improving the solid-to-liquid processing ratio from 0.17 g/mL to 0.23 g/mL [2]. Using a packed bed leaching column paired with an electrochemical reactor, this method selectively dissolved base metals while concentrating precious metals for separate, high-purity extraction [2].
Overall, base metal recovery from non-ferromagnetic e-waste fractions reaches about 90%, with metals like tin and zinc achieving complete extraction in chloride-based systems [2][9]. Advanced hydrometallurgical and electrochemical processes also boast recovery rates exceeding 98% for copper and 70% for gold [17]. Compared to traditional methods, these techniques operate more efficiently, requiring lower temperatures and fewer chemical inputs, making them a more sustainable choice for metal recovery.
Conclusion
Electrochemical recycling offers a powerful solution to the growing e-waste problem. In 2019 alone, the world generated 53.6 million metric tons of e-waste, yet only 17.4% of it was recycled [8]. This highlights the urgent need for recycling methods that not only recover metals effectively but also reduce environmental harm.
The benefits of electrochemical recovery are impressive. It delivers copper with 99.99% purity – far exceeding the 50–75% purity levels achieved through traditional smelting methods. Additionally, it processes 2.9 kg of e-waste per dollar, compared to just 1.3 kg for conventional black copper smelting [8][3]. On top of that, energy consumption can be cut by as much as 50% by replacing the oxygen evolution reaction with the oxidation of Fe²⁺ to Fe³⁺ at the anode [2].
Beyond performance, this method addresses the environmental drawbacks of older recycling techniques. It requires minimal reagents, regenerates leaching solutions for continuous reuse, and eliminates the toxic dioxin emissions associated with high-temperature smelting [8]. For businesses aiming to recover valuable metals while reducing environmental impact, electrochemical recycling checks both boxes.
As e-waste volumes continue to rise and natural metal reserves dwindle, electrochemical recycling is poised to play a crucial role in creating a sustainable system for electronics manufacturing. By reintroducing high-purity metals into production, it supports a circular economy that prioritizes resource efficiency and environmental responsibility. With its high recovery rates, superior metal purity, and reduced ecological footprint, this technology offers a practical and forward-thinking approach to managing our ever-growing digital waste.
This content is for informational purposes only. Consult official regulations and qualified professionals before making sourcing or formulation decisions.
FAQs
What are the environmental benefits of electrochemical recycling compared to traditional methods?
Electrochemical recycling stands out as a greener alternative compared to traditional methods like pyrometallurgy or chemical leaching. It works at much lower temperatures, which means it uses less energy – a win for both efficiency and the environment. Plus, it eliminates the need for many hazardous chemicals that could otherwise pollute ecosystems.
This approach also generates fewer harmful emissions and less waste, making it a cleaner choice for extracting valuable metals from electronic waste. By adopting this method, we can significantly reduce the environmental footprint of managing e-waste while recovering precious resources in a more responsible way.
What challenges arise when using electrochemical recycling to recover metals from e-waste?
Electrochemical recycling of metals from e-waste comes with its fair share of challenges. A significant hurdle lies in the intricate makeup of e-waste, which includes metals entangled with plastics, ceramics, and even hazardous substances. This complexity demands extensive sorting and pre-treatment before electrochemical methods can even begin. On top of that, precision is vital – factors like the electrolyte’s composition, current density, and temperature must be tightly controlled. Any misstep in these parameters can result in contamination or reduced recovery efficiency.
Taking the process from the lab to a commercial scale introduces additional obstacles, both economic and technical. The need for specialized equipment, ongoing management of electrolytes, and steep initial costs often make it less attractive compared to established methods like pyrometallurgy. Over time, other complications arise, such as electrode fouling, corrosion, and the disposal of spent electrolytes. These issues not only add to the technical complexity but also bring regulatory challenges, making long-term adoption a tough task.
Which metals can be recovered through electrochemical recycling?
Electrochemical recycling offers an efficient way to extract metals from electronic waste. It can recover precious metals like gold, silver, platinum, palladium, and iridium, along with base metals such as copper, nickel, and aluminum. On top of that, it retrieves rare-earth elements, which are critical for many modern technologies.
This approach stands out for its ability to minimize waste and preserve natural resources. By incorporating this recycling method, industries like electronics and manufacturing can adopt more environmentally conscious practices.





Comments are closed